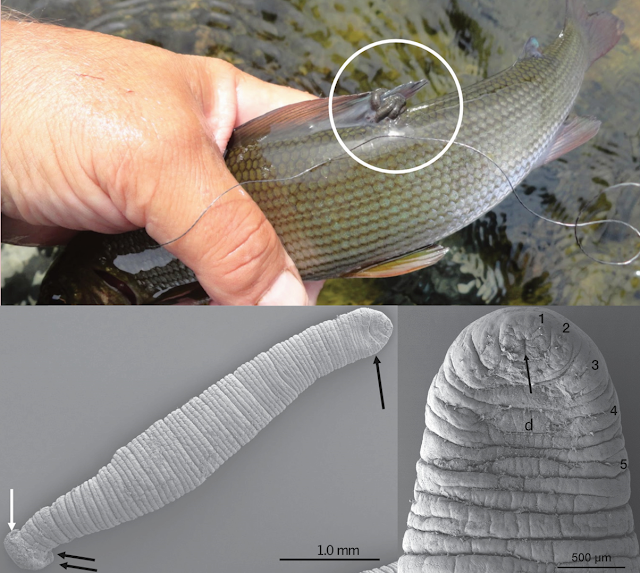
The bonefish is a popular recreational species for catch-and-release fishing. It is targeted by anglers using fly rods or light tackle, and requires some skills to do so as they're easily startled, and once hooked can put up quite a struggle. But if you are wading on a beach while fly fishing for bonefish, you might in turn become the target, because one of the bonefish's parasites may have its eyes on you too.
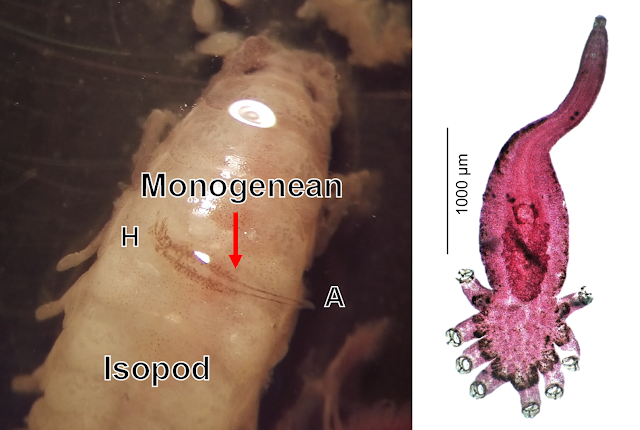
This blog has previously featured Cymothoidae isopods, which tend to be somewhat picky about what types of fish they parasitised But the isopod being featured in today's post isn't picky at all, in fact, when it comes to its next meal, and it doesn't always have to be a fish. Rocinela is a genus of isopods that belongs to the Aegidae family, and unlike the cymothoids which tend to stay on their hosts for extended periods of time, these isopods are temporary blood feeders, rather like land-dwelling leeches or bed bugs. On rare occasions, they can even feed on human blood. But adopting this kind of free-wheeling blood-sucking can open yourself up to becoming an unwitting carrier of many microscopic passengers.
The study we're looking at in this post investigated the health and microbes of bonefish at Belize. The scientists in this study captured bonefish around Ambergris Caye, and examined each fish for scars and ectoparasites (such as Rocinela), then collected some blood samples for genetic analyses. The scientists also analysed the blood present in the gut of the isopods they collected, to identify what kind of fish they had been feeding on. Genetic analyses of blood-suckers' meals have previously provided valuable insights into the hosts of ectoparasites.
Two of the three sites the scientists sampled from were frequented by Rocinela, and about 70 percent of the isopods they found on the bonefish had plump bellies that were full of blood. As expected, most of the isopods were filled with bonefish blood, but one of the Rocinela also had blood from a type of small killifish called the mangrove rivulus, and somewhat alarmingly, there was an isopod in the sample which had fed on human blood at some point.
What's even more interesting were the plethora of virus sequences that were found. Possibly because of its indiscriminate feeding habits, Rocinela has inadvertently picked up about 11 different types of viruses. Most of those were viruses that usually infect arthropods. One of them, XKRV-2, is related to a group of viruses which have been previously reported from a range of crustaceans, including parasitic isopods, so its presence was to be expected.
But one of the Rocinela also carried a less expected virus called XKRV-1, which is more related to a common genus of fish virus called Aquareovirus. None of the bonefish sampled had XKRV-1 in their blood, which means Rocinela has picked up the virus from one of other fish species that it had fed on. And rather than just being a transient, XKRV-1 has been persisting in the isopod's belly for a while - which is a common adaptation for vector-borne viruses such as those found in ticks and mosquitoes.
Given Rocinela can feed from a variety of fish, its payload of viruses may disembark into one of its hosts during feeding, so it could be transferring viruses between different species at sea. While Rocinela is also known to feed on humans, the likelihood of those fish viruses jumping into us is comparatively low - viruses that jump into humans tend to come from mammals and other warm-blooded animals, especially those that are evolutionarily closer to us, such as non-human primates. But a much bigger concern is that since Rocinela harbours so many different viruses and it is so indiscriminate about the type of hosts that it feeds on, it might end up acting like a transmission hub for viruses to jump from wild fish into aquaculture species.
Most studies looking at vector-transmitted diseases focus on land-dwelling arthropods such as ticks, fleas, and mosquitoes, but crustaceans like Rocinela and other parasitic isopods might be overlooked vectors that are providing a taxi service for pathogens under the waves.
Reference: