The deep sea is home to unique communities of organisms, and wherever there is life, there are parasites, and deep sea habitats such as hydrothermal vents with their multitude of species can provide the conditions to support parasites with complex, multi-host life cycles.
But not all such parasites are equal in their requirements, for example digenean flukes can be demanding prima donnas when it comes to the necessary hosts for their complex life cycles. Not only does the adult fluke needs a vertebrate host to live in, they also have an asexual stage that needs to go into a specific type of invertebrate, usually some kind of snail, and maybe even other small animal to act as go-betweens to carry the larval stage from the snail to the vertebrate host. So while habitats like hydrothermal vents are teeming with life - do they have what it takes to support those fastidious flukes?
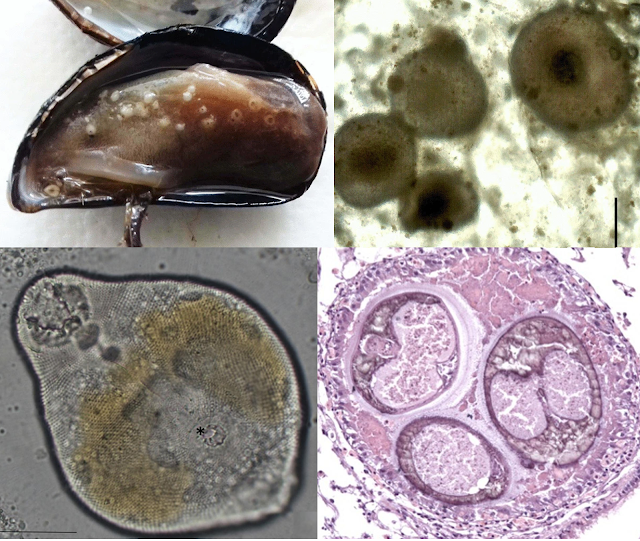
To answer that question, scientists from the Woods Hole Oceanographic Institution looked for parasites in samples of organisms collected from hydrothermal vent sites located about 2500 metres below sea level, along the East Pacific Rise. They ended up with a mixed bag of different animals composed of various fish and invertebrates, and when the scientists dissected those deep sea creatures, it turns out many of them were filled with all kinds of flukes at various stages of their life cycles. This range from adult flukes in the guts of vent fishes, to the sausage-like asexual stages in snails, to larval cysts embedded in the bodies of invertebrates, and even the short-lived, free-roaming cercaria stages crawling about in the samples.
Seven different types of flukes were present in the guts and gall bladder of vent fishes such as the pink vent fish (Thermarces cerberus) and viviparous brotulas (Thermichthys hollisi). And by sequencing selective sections of their DNA, the scientists were able to match those adult flukes with corresponding larval stages in a wide range of vent animals including shrimps, crabs, snails, and polychaete worms. For example, the fluke asexual stages found in glass limpets turned out to be a match with the adult flukes found in one of the vent fish.
In total, the researchers were able to identify three distinct genera - Biospeedotrema, Caudotestis, and Neolebouria - but they also found free-living cercaria stages of an unknown fluke. These cercariae have a stubby, sucker-like tail and while they superficially resemble the cercariae produced by the fluke which was found in glass limpets, DNA analysis shows that it does not genetically match with any of the other flukes in the samples. The life cycle and hosts of those peculiar cercariae are currently unknown, but their presence indicates that there many other infected hosts yet to be discovered at those hydrothermal vent communities.
So while this study has managed to fill some gaps in our knowledge about parasitism in the deep sea, there are still many mysteries. Digenean flukes need rich communities to complete their life cycles, and their presence at hydrothermal vents tells us that even though such vent sites are short-lived, these habitats can support a very rich community of different organisms. But many of these biodiverse habitats are under threat from a wide range of current and planned human activities. There is so much more that we need to learn about these biomes of the deep, and we also need to learn to value them, lest they become casualties in the face of ceaseless demands for minerals and other commodities.
Reference:
.png)