Galactosomum nagasakiense is a parasite that lives rent-free in fish's brain and give them "Trematode Whirling Disease" (TWD). There are actually quite a number of different fluke species that all infect fish brains, but unlike those other species where hundreds of individual flukes are needed to change how the fish behaves, all it takes is a single Galactosomum sitting snugly in the centre of a fish's brain to send it into a tizzy. Whereas other flukes manipulate the neurotransmitters in the brain of their host, Galactosomum takes a more blunt force approach, as the mechanical pressure exerted by their larval cyst causes the surrounding brain matter to degenerate or undergo necrosis as a by-product of their presence. So this fluke literally gives its fish host brain rot.
 |
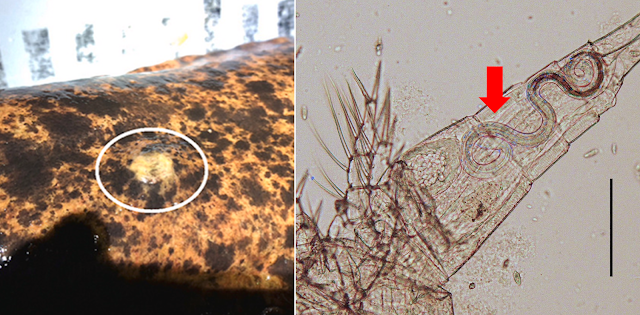
| From left to right: Line illustration of a Galactosomum nagasakiense cercaria, Photo of Galactosomum Type C, Photo of Galactosomum Type B (top) and Galactosomum cysts in the brain of a tiger buffer (bottom) with a close-up of the cyst (insert), photo of Cerithium dialeucum shell. Photos and illustration from Fig. 1, 4, 6, 7 of the paper and from the D-PAF (Database of Parasites in Fish and Shellfish) |
Galactosomum nagasakiense was first noticed in fish in the 1960s, and the adult fluke lives in the intestine of black-tailed gulls, who presumably appreciate the easy pickings that these brain rotted fish present as they swim in circles near the surface of the water. But where are the fish getting their flukes from? This is a important question because TWD can affect a wide range of fish from anchovies to kingfish, and is known for causing bouts of mass die-offs at fish farms among important aquaculture species such amberjacks and fugu (puffer fish).
Despite its impact, the full life cycle of G. nagasakiense was unknown until the publication of the study we'll be looking at in this post. Deciphering the complex life cycle of parasites can often be a labour intensive and thankless task which involves a fair amount of informed intuition and luck. Hence for many parasite species which are otherwise well-studied, often the one aspect about them which remains unknown is their full life cycle.
In this study, researchers conducted field sampling on the coast of Tsushima Island near a tiger puffer farm that regularly had cases of TWD. Knowing the typical life cycles of digenean flukes, the source of the infection would most likely be a snail, but which one? There are many sea snails living among the rocks on the coast of the island which are prime candidates as the source of G. nagasakiense. The researchers in this study ended up sampling 1314 cerithioid sea snails, most of which (798) belonged to a species named Cerithium dialeucum, which turned out to be the snail that was serving as crawling Galactosomum factories.
Infections were not common, only 15 out of the 798 snails they sampled were shedding Galactosomum larvae, but they made up for their rarity with productivity. During their peak emergence period which lasted two to three weeks, each snail can pump out 3000 of those wriggling Galactosomum larvae per day, though this number declines to a steady (but persistent) trickle for another ten weeks. In total, each infected snails can release 16000 Galactosomum larvae into the surrounding waters, made possible by the prolific asexual stages of the parasite which has taken over the snail's internal organs.
The free-swimming larval stage of digenean flukes come in all kinds of shapes and sizes, but Galactosomum stands out for having a thick, rippled tail which is about ten times as long as the larval fluke's actual body. When these wrigglers leave the snail, they swish their tail in a figure 8 motion that would attract the attention of any curious fish.
The researchers also discovered that G. nagasakiense was not the only species of Galactosomum in those snails, they found two other species of big-tailed cercariae, one of which has a sucker-like structure on its massive tail. It is likely that those species also infect fish, but it is unclear whether they also burrow into fish brains the way G. nagasakiense does. In any case, the discovery of these snails as the source of infections, can provide aquaculture managers with ways to limit or control TWD, such as situating the fish farms away from habitats where those snails are likely to be found.
The influence of parasites on their hosts are often overlooked because they are hidden out of sight inside of their hosts, but their impacts cannot be ignored. In this case, all it takes is a tiny fluke to cause some serious headaches for a whole lot of fish and fish farmers.
Reference:
Sugihara, Y., Iwasaki, R., Miyazaki, H., Shirakashi, S., Itoh, N., Nakano, T., Takano, T. & Ogawa, K. (2026). Elucidation of the life cycle of Galactosomum nagasakiense (Heterophyidae), the causative parasite of trematode whirling disease in marine fish, with discovery of congeneric species in the gastropod first intermediate host Cerithium dialeucum. Parasitology International 111:103190.