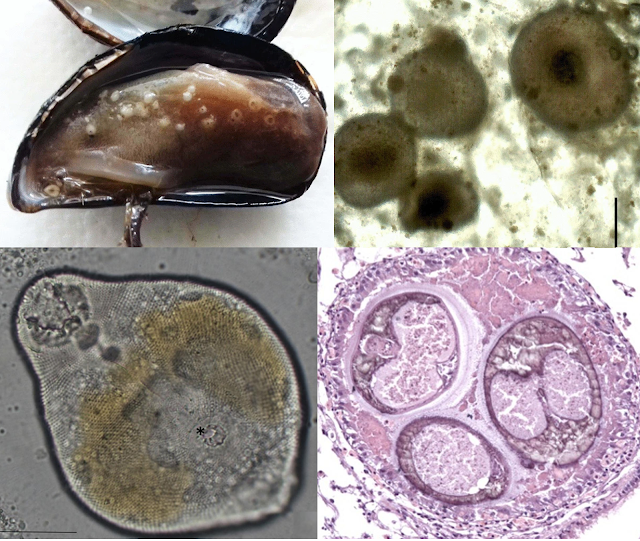
The bay scallop (Argopecten irradians) is a highly prized shellfish, but it has suffered through a rough history from overharvesting, habitat loss, and natural enemies. This culminated in a massive population decline in the 1980s that led to the closure of all its fisheries across certain regions along North Carolina. With the depletion of its wild populations, efforts are being made to raise the bay scallops in aquaculture to meet demands. But now a new woe has fallen upon this besieged bivalve with the appearance of a never-before-seen parasite.
 |
| Left: Bay scallop infected with Saccularina, the red arrows indicating the parasite's sporocysts in the gills. Right: the cercaria stage of Saccularina which is a "cystophorous"-type cercariae. From Fig. 1 and 2 of the paper |
In 2012, a researcher started noticing a gill-dwelling parasite in both caged and wild bay scallops along the coast of North Carolina, and later at the Gulf Coast of Florida. These parasites are readily visible in the shellfish’s gills as they become swollen with the parasite's presence. Examination under a microscope revealed the parasites to be a species of parasitic fluke which is using the scallop for the asexual stage of its life cycle. Essentially, these trematode flukes are converting the shellfish into a parasite clone factory that pumps out a stream of free-swimming larvae to infect the next host in the life cycle.
Given such an operation consumes a lot of the host’s resources, this can interfere with the scallop’s growth and survival, and thus it is a major concern to the scallop fisheries in North Carolina. The key to managing any parasitic infection is an understanding of its natural history and life cycle, and unfortunately, given its relatively recent discovery, the life cycle of this parasite is almost entirely unknown. However, related fluke species can give us some clues, and as it turns out, this bay scallop parasite is no ordinary fluke.
First of all, DNA analysis showed that one of this fluke's closest relatives is Saccularina magnacetabula, a trematode species found on the other side of the world in Australia. While it is genetically similar enough to the bay scallop parasite for them both to be in the same genus (Saccularina), there is enough geographical and genetic distance between them that they are clearly different species. Furthermore, instead of scallops, S. magnacetabula infects the Sydney cockle (Anadara trapezia) as the chosen bivalve for its asexual stage. As for the parasite's next stops, it's a multi-part journey involving tiny crustaceans, followed by a type of smallish Australian fish called whiting (Sillago sp.), and finally the adult fluke completes its life cycle nestled in the fin membranes of the giant herring (Elops hawaiensis).
Saccularina magnacetabula, and by extension, the bay scallop parasite, belongs to a family of flukes called Didymozoidae - a flukey group of flukes with some very unusual anatomy and habits. While the adult stages of most trematodes are generally leaf-shaped, didymozoids come in all kinds of shapes and sizes. And they are found in a wide range of different bony fishes all over the world, mostly marine species. Additionally, instead of living in the final host’s gut like most flukes do, didymozoids cram themselves into all kinds of nooks and crannies such as the muscles, the gills, or even the fin membranes as is the case for S. magnacetabula.
While there are many known species of didymozoids, the life cycles for most of them are a mystery, with the hosts for the asexual stage known only for a few species. But those handful of species alone showed didymozoids to have quite the eclectic range. Aside from bivalves like cockles and scallops, the asexual stage of other didymozoids infect snails, but not just any regular snails - one species is known to use pelagic sea snails (which are also called “sea elephants”), while another species infects worm snails which are peculiar sea snails with twirly shells that encrust on rocks and other hard surfaces.
So, based on the information above, we can make some inference about the likely source of the bay scallop parasite. It’ll have to be some kind of predatory sea-dwelling fish harbouring the adult stage of the fluke, and given S. magnacetabula completes its life cycle in the giant herring, the bay scallop parasite is most likely completing its life cycle in some kind of predatory herring-type fish in the region, which means ladyfish or Atlantic tarpon.
While we may have some clues about the bay scallop parasite’s life cycle, how they might have gotten there is more of a mystery though. This parasite was first seen in bay scallops in 2012, but if the disseminator of this parasite is really a local fish species such as the tarpon, why has it only been noticed now? Whatever its origin turns out to be, it seems the bay scallop parasite is not ready to give up its (many) secrets.
Reference:
Boggess, H. F., Varney, R. L., Freshwater, D. W., Ben-Horin, T., Preister, C., McCurry, H., Wilbur, A. E. & Buck, J. C. (2024). A newly discovered trematode parasite infecting the bay scallop, Argopecten irradians. Aquaculture 589: 740960.